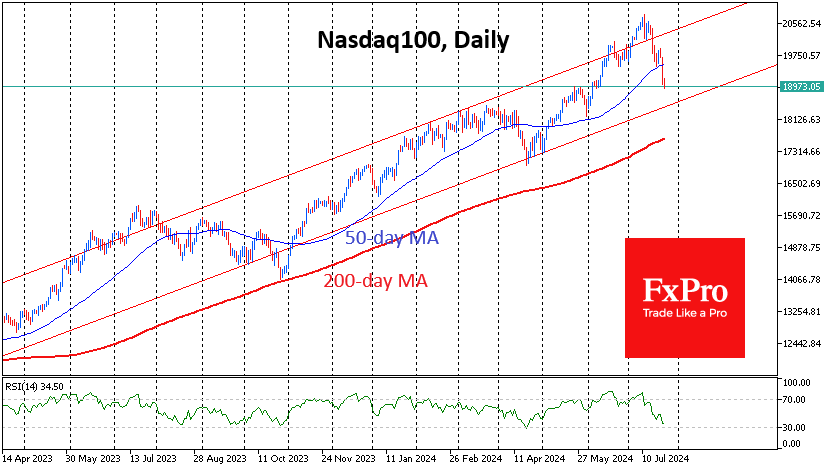
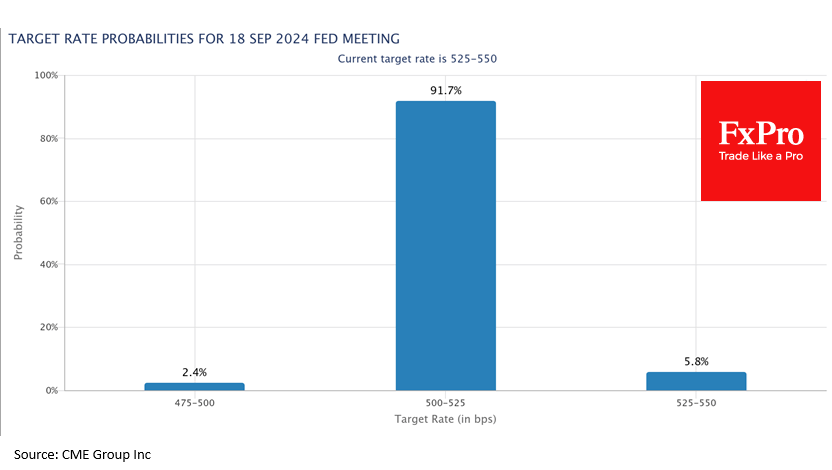
Wall Street analysts are cautiously optimistic on J&J’s ‘one-dose’ Covid vaccine
January 11, 2021 @ 12:33 +03:00
Health care systems around the world are struggling to cope with rising numbers of Covid-19 infections as they race against the clock to vaccinate the vulnerable. The three vaccines currently approved for use by major Western economies all require two separate jabs and given supplies are limited, governments are considering contentious tactics like stretching the length of time between doses to get at least one dose to as many people as possible.
A one-shot vaccine could significantly improve our ability to fight the virus — and we may have one soon. Johnson & Johnson is expected to deliver preliminary late-stage trial results for its one-dose Covid vaccine candidate by the end of January. If its jab is proven to be safe and effective, the company aims to deliver at least 1 billion doses by the end of the year.
The J&J vaccine was developed by the company’s Belgian unit, Janssen Pharmaceutica, and is based on viral adenovirus vector technology, the same approach used to create the University of Oxford-AstraZeneca vaccine. This type of shot is easier to scale up than those developed by Pfizer-BioNTech and Moderna which are based on messenger RNA technology.
Health care analyst Adam Barker at Shore Capital said in an email to CNBC last week: “The J&J vaccine is more like the AstraZeneca vaccine, but it uses only one dose. So we know this approach works (viral-vector) and it targets the spike protein. We know that target works too. But, we’ll have to see what one dose does.”
Wall Street analysts are cautiously optimistic on J&J’s ‘one-dose’ Covid vaccine, CNBC, Jan 11