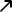European Union begins review of Russia’s Sputnik Covid vaccine
March 04, 2021 @ 17:16 +03:00
The European Medicines Agency said Thursday it will start assessing the Russian coronavirus jab, Sputnik V, as the bloc looks to speed up its vaccination program. “EMA will assess Sputnik V’s compliance with the usual EU standards for effectiveness, safety and quality. While EMA cannot predict the overall timelines, it should take less time than normal to evaluate an eventual application,” the regulator said in a statement.
The EMA is using a rolling review to study the data for the jab developed in Russia. This allows the European medical authority to assess its efficacy as it receives all the necessary information, before the vaccine maker can apply for a formal authorization. By analyzing the studies ahead of the application, the EMA’s potential approval could come quicker than usual.
The news comes after a number of European countries indicated that they could start administering Sputnik V, bypassing the regulator. Slovakia and Hungary have already ordered doses of the Russian jab while the Czech Republic and Austria are considering the vaccine.
In January, German Chancellor Angela Merkel said she was “open” to the idea of producing Russia’s coronavirus vaccine in the European Union. European Commission President Ursula von der Leyen in February questioned why Russia was offering up so many of their vaccines when its own population is yet to be fully vaccinated.
European Union begins review of Russia’s Sputnik Covid vaccine, CNBC, Mar 4