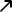Officials rush to defend AstraZeneca Covid vaccine after UK, EU blood clot guidance
April 08, 2021 @ 22:21 +03:00
The British government and health experts in the country have rushed to defend the coronavirus vaccine developed by AstraZeneca and the University of Oxford following concerns over a possible link to blood clots. On Wednesday, the U.K.’s health and vaccine regulators issued a change of guidance over who should receive the shot. They now recommend that anyone under the age of 30 should receive an alternative vaccine amid concerns that it could, in rare circumstances, lead to a serious blood clot.
Following a safety review of the AstraZeneca vaccine, sparked by concerns over reports of rare blood clotting disorders in a small number of vaccinated individuals, both the U.K. and European medicines regulators (the MHRA and EMA, respectively) stressed that the benefits of the jab still outweighed the risks. However, amid concerns that the reputation of the vaccine could be damaged further, experts have rushed to defend it – and one Twitter user commented that officials appeared to have gone into “damage limitation” mode.
On Thursday, the U.K. health secretary stressed that the risk of a blood clot after receiving the AstraZeneca Covid vaccination is about the same as on a long-haul flight. He said the safety measures surrounding the vaccine were robust and enabled regulators to “spot this extremely rare event.” Meanwhile, U.K. Prime Minister Boris Johnson, who has received a first shot of the vaccine himself, said that “the best thing people should do is look at what the MHRA say, our independent regulator – that’s why we have them, that’s why they are independent.
Officials rush to defend AstraZeneca Covid vaccine after UK, EU blood clot guidance, CNBC, Apr 9