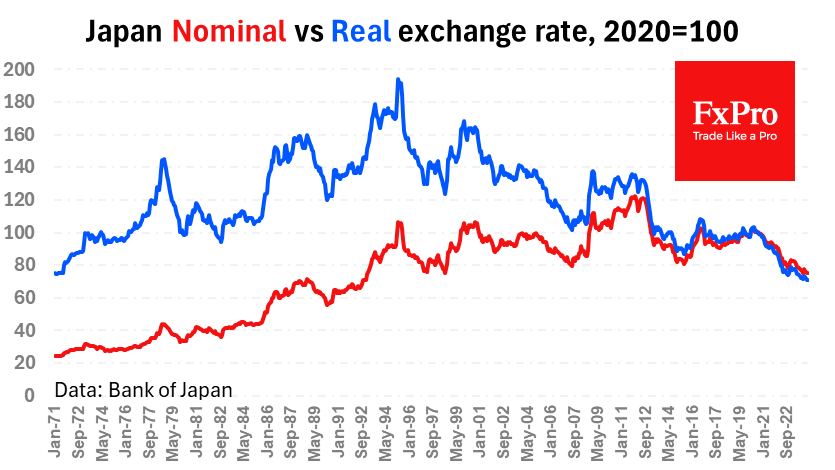
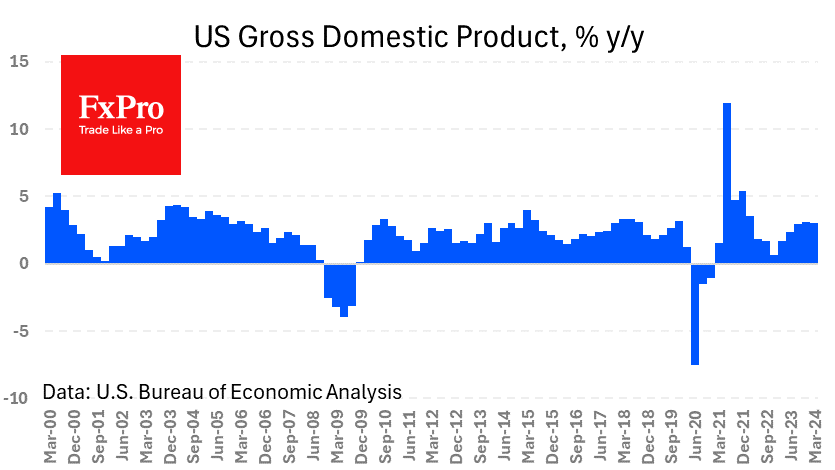
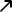A quarter of Americans are hesitant about a coronavirus vaccine – Reuters/Ipsos poll
May 21, 2020 @ 17:35 +03:00
A quarter of Americans have little or no interest in taking a coronavirus vaccine, a Reuters/Ipsos poll published on Thursday found, with some voicing concern that the record pace at which vaccine candidates are being developed could compromise safety.
While health experts say a vaccine to prevent infection is needed to return life to normal, the survey points to a potential trust issue for the Trump administration already under fire for its often contradictory safety guidance during the pandemic.
Some 36% of respondents said they would be less willing to take a vaccine if U.S. President Donald Trump said it was safe, compared with only 14% who would be more interested.
Most respondents in the survey of 4,428 U.S. adults taken between May 13 and May 19 said they would be heavily influenced by guidance from the Food and Drug Administration or results of large-scale scientific studies showing that the vaccine was safe.
Less than two-thirds of respondents said they were “very” or “somewhat” interested in a vaccine, a figure some health experts expected would be higher given the heightened awareness of COVID-19 and the more than 92,000 coronavirus-related deaths in the United States alone.
“It’s a little lower than I thought it would be with all the attention to COVID-19,” said Dr. William Schaffner, an infectious disease and vaccine expert at Vanderbilt University Medical Center in Nashville. “I would have expected somewhere around 75 percent.”
Fourteen percent of respondents said they were not at all interested in taking a vaccine, and 10% said they were not very interested. Another 11% were unsure.
Studies are underway, but experts estimate that at least 70% of Americans would need to be immune through a vaccine or prior infection to achieve what is known as “herd immunity,” when enough people are resistant to an infectious disease to prevent its spread.
Trump has vowed to have a vaccine ready by year’s end, although they typically take 10 years or longer to develop and test for safety and effectiveness. Many experts believe a fully tested, government-approved vaccine will not be widely available until mid-2021 at the earliest.
Among those respondents who expressed little or no interest in a coronavirus vaccine, nearly half said they were worried about the speed with which they are being developed. More than 40% said they believe the vaccine is riskier than the disease itself.
Overall, 84% of respondents said vaccines for diseases such as measles are safe for both adults and children, suggesting that people hesitant to take a coronavirus vaccine might reconsider, depending on safety assurances they receive.
A quarter of Americans are hesitant about a coronavirus vaccine – Reuters/Ipsos poll